What Is Graphite?
Natural graphite is a mineral composed of graphitic carbon (Cg); it is mostly obtained from primary sources, i.e., from mining. Natural graphite should not be confused with synthetic graphite that is produced from calcined petroleum coke and coal tar pitch that have lower crystallinity.
Natural graphite is classified as:
- Crystalline vein graphite
- Microcrystalline (amorphous) graphite
- Flake graphite

Types of Graphite
Flake Graphite
Flake graphite can be found in metamorphic rocks and have a flake or flat particle form of various sizes. It is usually characterised as fine (-100 to +200 mesh), medium (-80 to +100 mesh), large (-48 to +80) or jumbo (+48 mesh) and can contain up to 90-97% carbon. Carbon concentration in flake graphite deposits ranges from about 2% to 40% Cg. After mining, flake graphite ore is usually beneficiated via froth flotation to extract flake graphite. The result is a concentrate with 80%–90% graphite content.
Microcrystalline Graphite
Microcrystalline graphite, also referred to as “amorphous”, is the least graphitic among the natural graphites. Amorphous graphite can be found as tiny particles (-200 mesh, or <75 microns) in metamorphic anthracite or in carbonaceous coal beds. The graphite content varies from 25% to 85% according to the geological environment.
Highly Crystalline Graphite
This type of graphite may be referred to as “vein” or “lump” graphite. It usually occurs as veins or filled fissures in igneous and crystalline metamorphic rocks. The only commercial deposits are in Sri Lanka, where veins are said to be up to 3 metres thick and mined at depths of 30 to 650 m. The ore is mined by hand by artisanal miners, resulting in a high-grade product with more than 90% graphitic carbon.
Applications
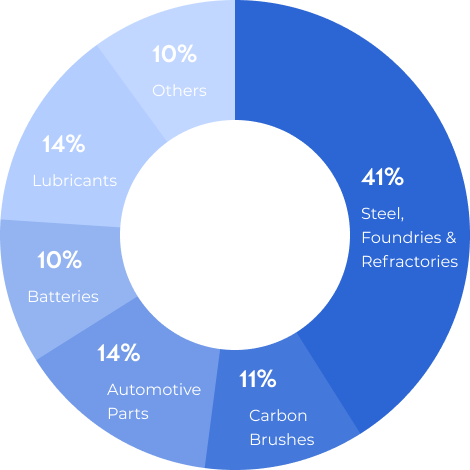
Natural graphite is a refractory material with a high melting point of 3650 °C, and it is a good conductor of heat and electricity. Some of the applications for graphite include: batteries, lubricants, refractories, coatings and paint, metallurgy and moderator rods in nuclear power plants, among other applications.
Anode for electric vehicles
Graphite is currently an essential element for the production of lithium-ion batteries. Global demand for natural graphite and spheroidal graphite in battery grade for anodes is growing rapidly and will increase exponentially from 2022. The batteries are mainly required for consumer electronics and electric vehicles.
In addition to the steel industry, Graphite is also an important raw material for lighter materials such as carbon fibre, reinforced plastic used in automobiles and in the manufacture of components for aircraft.
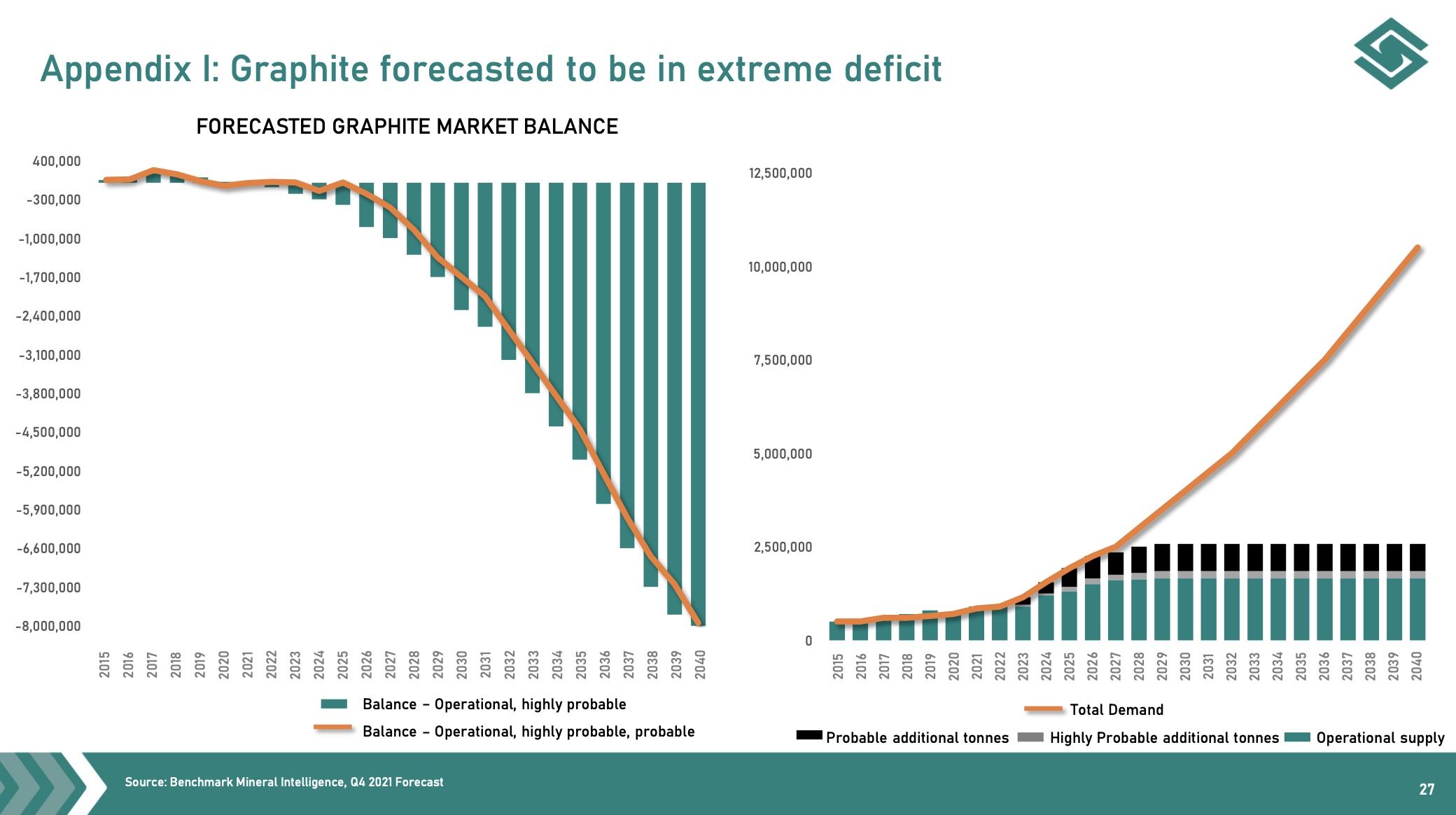
Graphite anode plants are mainly located in China and produced about 5-10,000 tons per year. Global demand is increasing. Total current production of natural graphite is 1.1 million tonnes. As internal combustion engine vehicles are phased out, graphite demand for EV batteries alone could increase to 5 million tonnes, creating the opportunity for dozens of new graphite mines to open in the next 5 to 10 years.
From 2020 onwards, four new anode megafactories plan to produce 60,000-100,000 tonnes per year. It is foreseeable, and also the prediction of many experts, that there will be supply problems of raw materials for these battery megafactories in the next 2 years. There are 76 lithium-ion battery megafactories. That’s 35 more than a year ago. 46 more are under construction. More will follow. The amount of graphite that will be needed in these megafactories is millions of tons. Right now, the anode space is about 165,000 tonnes per year, but we’re going to need well over 1.6 million tonnes per year by 2030 if all these plans come to fruition. China accounted for 62% of natural graphite production in 2020 and is now in the process of rationing production capacity due to resource depletion and environmental concerns.
Lubricants, grease
Graphite is used as a dry, solid lubricant and has become one of the traditional and most important dry lubricants worldwide. It is used, for example, as a lubricant for forging tools, stampings made of steel, titanium, stainless steel, special superalloys, as well as in die forging and the hot and impact extrusion processes.
Graphite has excellent lubrication, release and demolding properties. It has a high hiding power and guarantees excellent tool wettability. The use of very pure graphite and highly refined oils results in low engraving and tool wear and low residues.
Refractories used in steel, crucibles, etc.
The electrical conductivity of graphite is about a hundred times higher than that of general non-metallic minerals. In steel production, graphite is used as a protective agent of the steel ingot and for lining the metallurgical furnace.
Graphite is also used in aluminum production, as an alloying component in cast iron and in the semiconductor industry.
Graphite, in the form of flakes or expanded graphite, offers the possibility of developing metal-graphite composites. Depending on the content and orientation of the incorporated graphite, it can be used as heat spreader material for thermal management, as a passive damping element for plant and mechanical engineering, or as bearing material for slowly rotating plain bearings. By combining materials, properties such as strength, thermal conductivity, damping factor, the coefficient of thermal expansion and Young’s modulus can be specifically matched to the intended application. Powder-metallurgical production allows the carbide content to be controlled in a targeted manner and graphite contents of up to 90% by volume to be realised without pores. Mechanical finishing is therefore possible even with very high graphite contents.
Brake lining
Graphite is used in the production of brake pads. The use of graphite powder has the main objective of solid lubrication and friction stabilisation and can serve both as a friction agent and as a binder for brake pads.
Pencils
Graphite has a long tradition in the production of pencils. Since the 19th century, pencil lead has been fired from a mixture of graphite and clay. While clay gives the hardness and paleness, graphite gives a pencil the properties that can best be described as blackness and softness.